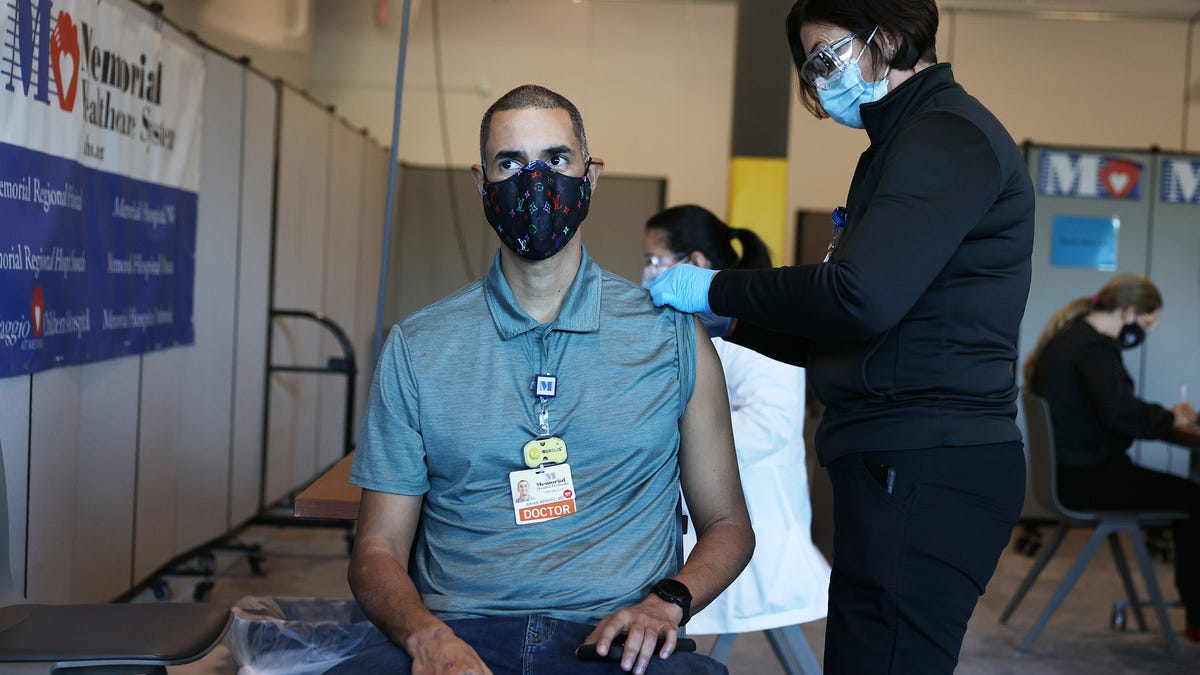

As the first Covit-19 vaccines are released across the United States, some of us are chanting “Get me an injection now,” while others are curious about the side effects. I approached two dozen scientists and public health professionals to see how they felt about these vaccines. I asked them if they plan to get it when a vaccine is available, as well as what concerns, if any, they may have and what they will say to those who are not worried about the vaccine.
Across the board, they all expressed a desire to get vaccinated as soon as possible, believing that the clinical trial process has done the job of testing these vaccines for public use.
On Monday, the first doses of Pfizer and Bioentech’s FDA – approved Govt-19 vaccine reached the U.S. population. By this weekend, Moderna’s similar MRNA vaccine is expected to receive its own emergency use approval. Both vaccines are highly effective in preventing symptoms from Govt-19 (more than 90%) and are poised to reverse the tide of an epidemic that has killed more than 1.6 million people worldwide and more than 300,000 Americans in less than a year. .
Still others worry about getting vaccinated. Some of these fears can be found in the false myths about how these or other vaccines work (MRNA vaccines do not alter our body’s DNA, for example). Some may wonder whether political pressure has encouraged scientists or public health organizations to take shortcuts.
“I’m a leading healthcare professional who communicates regularly with many patients with Covit-19 positives. Kritika Kuppalli said.
G / O Media may receive a commission
Is the vaccine available? “Yes, of course,” said Robert Amler, dean of the New York School of Medical Sciences and Practice and former chief medical officer at the CDC’s Toxicology and Disease Register. “Phase 3 trials require a very serious study of the effectiveness and safety of a vaccine.”
Although these vaccines were developed at the time of registration, this speed has come from the avoidance of logistics barriers, i.e. delays of several years between trials — not by taking shortcuts such as studying a small number of people. In fact, more than 70,000 volunteers in the United States alone have joined the third phase trials of Pfizer / Bioendech and Moderna – a larger number than the third phase trials of a new drug. Although these are the first MRNA vaccines to reach the general public, this type of vaccine Studied In small trials of animals and people for more than a decade, a good safety track record so far. The FDA, despite some misconceptions during epidemics, exists again and again Rebuked The Trump administration is trying to interfere in its testing process.
Importantly, these vaccines seem to work to prevent people from becoming seriously ill.
Peter Hodes, dean of the National Tropical Medical School in Baylor Medical College in Houston, One of the scientists trying to develop a vaccine for Govit-19. Vaccination of his team Being now Tested In India, it is very effective due to its low cost. He said he hopes the vaccines will be available to the public soon.
“The reason is that all of the antiviral vaccines, including ours, work by inducing virus-neutralizing antibodies, and we know that VNAs are the best guarantee to keep you and loved ones out of the ICU,” he said.
This is not to say that there are no vaccines Side effects, Which includes things like headaches and fatigue, or no lasting questions that take time to answer.
“The data available so far do not bother me, but I would like to see more about the duration of immunity, the characteristics of individuals who have been vaccinated and the long-term safety follow-up,” said Walter Orenstein, former director and current director of the CDC’s National Immunization Program. Associate Director Of the Emory University Vaccine Center. Orenstein has already been included in the third phase of the investigation into Moderna, which is still in its infancy. He said he would get the real deal soon if he knew he had received the placebo.
Some of the experts I spoke to were concerned about the release and distribution of these vaccines.
Angela Rasmussen, a virologist with the Global Health Sciences and Safety Center for Georgetown University Medical Center in Washington, D.C., said: “It is unclear how many doses will be available after each initial 20 million doses.
Brandon Brown, an epidemiologist at the University of California at Riverside, says any vaccine should be used with caution in high-risk groups if given a low supply. The CDC has already laid down guidelines for the distribution of these first levels, prioritizing the elderly living in leading healthcare facilities and nursing care facilities. But each state will have its own distribution plan, and there are already allegations that some groups may come Cut evenly. On Monday, the White House Reverse Its staff plans to get the vaccine in front of the general public.
“No one can buy their front line unless they are in high risk,” Brown said.
Aside from supply and distribution barriers, there is concern that many will reject the vaccine when it is finally available. Voting varies by question, but Americans make up 40 percent Is still embarrassing About getting a Govt-19 vaccine. Some of this reluctance has been triggered by the propaganda of the anti-vaccine movement Continues Avoid being checked on websites and social media like Amazon. But there is also a very understandable feeling Distrust In the medical profession among color communities, it fuels higher rates of vaccine reluctance compared to the general population.
Clinical trials have, for the most part, done a good job of involving a wide variety of volunteers, which is important not only scientifically, but also to rebuild that confidence. There are also states like New York Insists Contributions made by color scientists in developing these vaccines, as well as color health workers Volunteer Must be some of the first people to see getting shots outside of a test.
Many of the scientists I spoke to agreed that there would not be an easy way to convince everyone on the fence about a vaccine.
“People of color, for example, prefer to acknowledge and address the history of exploitation medical research and health inequalities. Those skeptical of this process generally have to ask how the disciplinary process works,” said Rasmussen. . “
Still others can be trusted to ask for someone’s empathy.
“I will talk to them about the importance of not only their health, but also those in the community, their friends, family, and neighbors,” said Kuppally, who was hesitant about a vaccine when asked what he would say to people in his own life.
Talking to these scientists, now and throughout the year, I felt like developing a safe, effective vaccine for Govt-19, relatively speaking. The next step in getting enough people to do this will require a lot of work.