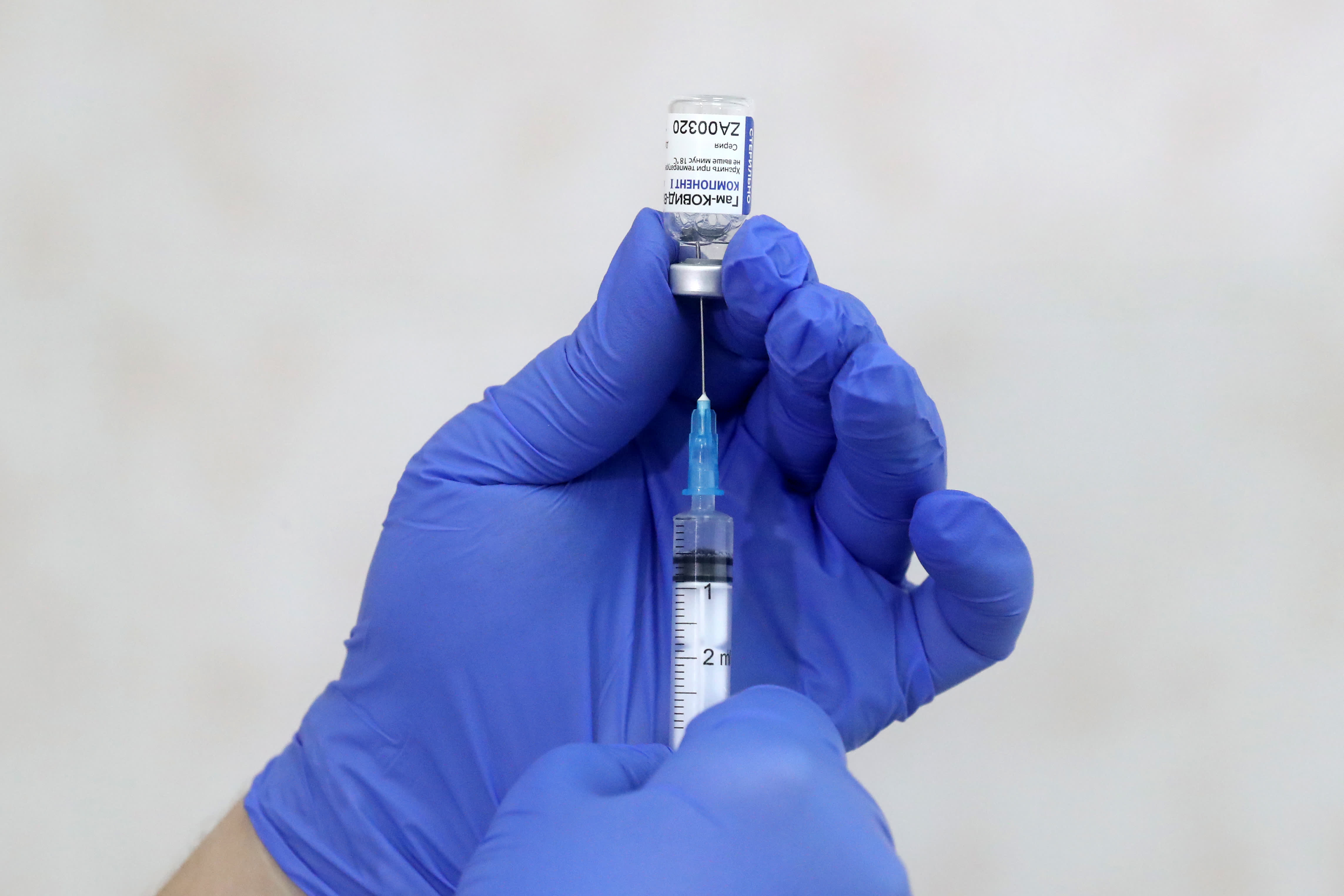
A medical worker fills a syringe with the Gam-COVID-Vac vaccine (branded Sputnik V) in Butovo, south of Moscow.
Sergei Savostyanov | TASS | Getty Images
DUBAI, UAE – Russia’s Sputnik V Covid-19 vaccine gained momentum on Thursday as Hungary and the United Arab Emirates became the top countries in the European Union and the Gulf region, respectively. to register the shot for emergency use.
Hungary’s decision was confirmed by President Viktor Orban’s spokesman, who said that if the country agrees to a shipping agreement with Moscow, it will become the first European Union country to receive the vaccine. This happens when the country’s cases have dropped from a maximum of more than 6,000 a day in early December to less than 2,000 a day.
“This decision is very important, as it shows that the safety and efficacy of the vaccine above 90% are highly valued by our partners in Hungary,” Kirill Dmitriev, head of the Direct Investment Fund, said in a statement. of Russia.
EU drug regulator has not yet approved the Russian sting, although German Chancellor Angela Merkel gave more hope to Sputnik on Thursday, suggesting the German vaccine regulator could advise Russia on the approval process. the EU. RDIF has submitted Sputnik for EU registration and awaits its review in February.
UAE approval comes amid a dramatic rise in infections
The approval of the United Arab Emirates comes amid a record increase in cases in the small Gulf Sheikh, which has stood out internationally for hosting tourists and fully reopening its economy in late summer last year.
Confirmed cases of coronavirus have tripled in a span of about three weeks, leading the UAE authorities to suspend non-essential surgeries in hospitals and “entertainment activities” in their lively hotels and restaurants for a few days. after assuring the country that the virus was under control.
The UAE’s daily case count reached a record high of 3,529 on Thursday, well above neighboring Gulf countries, where recorded infections are below 500 per day.
An Emirati man, wearing a protective mask, walks into the al-Barsha Health Center in the Gulf of Dubai Emirate on December 24, 2020.
GIUSEPPE CACACE | AFP via Getty Images
Sputnik V will be the third vaccine to be rolled out in the United Arab Emirates after China’s Sinopharm vaccine and the Pfizer-BioNTech jab developed by the United States and Germany were made available to the public in December. The country, with about 10 million, is carrying out what its government says is the second fastest national vaccination campaign in the world after Israel, per capita, and intends to inoculate half of the country’s residents. country in late March.
“The decision is part of the UAE’s comprehensive and integrated efforts to ensure increased levels of prevention,” the country’s health ministry said on Thursday about Sputnik’s approval. “The results of the study have demonstrated the effectiveness of the vaccine in triggering a strong antibody response against the virus, its safety for use, and its compliance with international safety and efficacy standards.”
Lack of final phase test data
The approvals came despite detailed unpublished research data on the results of human vaccine test 3. The capital of the United Arab Emirates, Abu Dhabi, began testing Phase 3 of Sputnik V earlier this month, but has not released any data. The RDIF says 1,000 emirate volunteers have received their first dose.
Sputnik V, which its developer, the Gamaleya Research Institute, says is 91% effective after two doses, has been in use all over Russia for months. Scientists have expressed concern over what many have described as a hasty release of the vaccine, lit in green for mass use in Russia before phase 3 trials are completed.
As a first step in the largest vaccination campaign in Argentina’s history, front-line health workers receive the Russian Sputnik V vaccine against coronavirus.
Patricio Murphy | SOUP Pictures | LightRocket | Getty Images
The analysis of the phase 1 and 2 trials of the vaccine was published in the peer-reviewed medical journal The Lancet in September, which said the first results showed no significant negative side effects, but more studies were needed.
“The results of the phase III clinical trials are expected to be published shortly,” according to the official Sputnik V website.
Prior to Thursday’s announcements, it had been approved for emergency use in 9 countries and territories outside Russia: Algeria, Argentina, Bolivia, Belarus, Serbia, Venezuela, Paraguay, Turkmenistan and the Palestinian territories.