Editor’s Note: Find the latest COVID-19 news and guidance at the Medscape Coronavirus Resource Center.
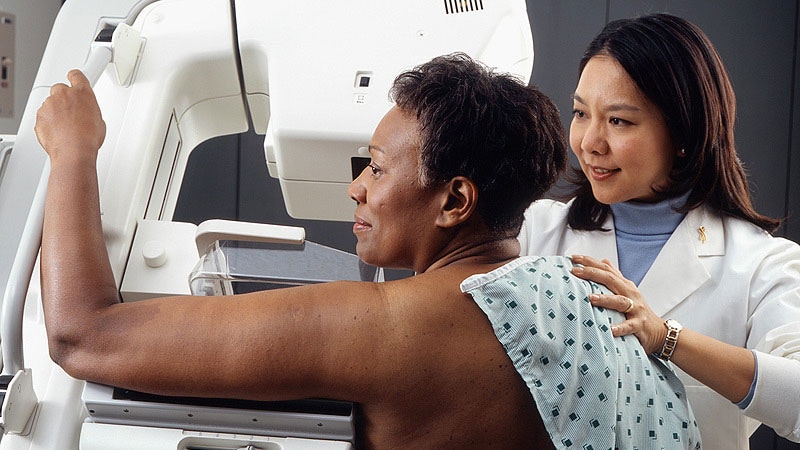
Women have reported axillary lymphadenopathy or swelling under the armpit after receiving the Pfizer-BioNTech and Modern COVID-19 vaccines, but it is also a common symptom of breast cancer.
Therefore, physicians should take into account the recent history of COVID-19 vaccination in the differential diagnosis of patients with unilateral axillary lymphadenopathy, according to a new article.
“We observed an increasing number of patients with swollen lymph nodes on one side / armpit who presented for routine mammography or ultrasound detection, and some women who actually felt these swollen lymph nodes,” said author Katerina Dodelzon, MD, Assistant Professor of Clinical Radiology, Weill Cornell Medicine, New York City.
“Historically, swollen lymph nodes on one side are relatively rare and are uncommon on screening mammography (seen only between 0.02% and 0.04% of the time) and are a sign that warns a radiologist. which excludes the presence of breast malignancy, ”she added.
In an article published in Clinical image, Dodelzon and colleagues describe four cases related to women who received a COVID-19 vaccine and then sought a breast exam. In describing these cases, the authors attempted to “inform the medical community to consider this diagnosis benign and self-resolving in the context of what may be an alarming presentation of unilateral axillary adenopathy.”
They hope to decrease unnecessary biopsies and help reassure patients.
Adenopathy has been reported in association with other vaccines, such as the Calmette-Guérin bacillus vaccine, influenza vaccines, and human papillomavirus vaccine, said Jessica WT Leung, MD, president of the Society of Breast Imaging (SBI).
“It’s too early to tell if there’s anything different about COVID-19 vaccines,” said Leung, who is also a professor of diagnostic radiology and vice president of breast imaging at MD Anderson Cancer Center at the University of Texas, Houston. Texas.
“The two vaccines currently in use – Pfizer and Moderna – are mRNA vaccines and it is unknown whether these will give a stronger immune response,” he said. “If Johnson & Johnson and AstraZeneca vaccines are available, it will be interesting to see if they get such a strong response, as they are not mRNA vaccines. At this time, we have no data to say so in one way or another. another. “
Leung also noted that these latest vaccine reactions may be attracting more attention because “it is related to COVID-19 and everything related to COVID-19 is more striking.
“It may also be more noticeable because of the large number of people who get vaccinated in a short period of time in an effort to contain the pandemic, and that is not the case with other vaccines,” he said.
New SBI recommendations
The SBI recently issued recommendations to doctors to follow up on women who experience axillary lymphadenopathy and have recently been vaccinated on the same side where adenopathy occurs for a few weeks to see if the lymph nodes return to normal instead. to undergo a biopsy.
“Many practices are routinely investigating the history of recent vaccination and on which side it was administered,” Dodelzon said. He stressed that women should feel empowered to share this story if not asked.
“Let your mammography technologist or mammographer know that you have recently been vaccinated and on which side, you will provide the breast image with a more accurate context for interpreting the results,” he said.
In addition, the SBI recommends that, if possible, women schedule a routine screening mammogram before the first dose of the COVID-19 vaccine or 4 to 6 weeks after the second dose to avoid a false-positive finding.
“We want to emphasize that screening mammography is very important and, if possible, programming it around the vaccine,” Leung commented. “But that may not be possible, as most of us have no choice when it comes to getting the vaccine.”
If neither a mammogram nor a vaccine can be rescheduled, Leung recommends that women report to the facility that they have recently received a COVID-19 vaccine. “Currently, we recommend follow-up in 4 to 12 weeks,” he said. “The swelling could subside sooner, maybe even in a week or two, but it’s generally recommended to wait at least 4 weeks to catch most women.”
Differences between vaccines?
The frequency with which axillary adenopathy occurs as a side effect differs with the two COVID-19 vaccines, according to reports from the Centers for Disease Control and Prevention (CDC).
For the Modern vaccine, ipsilateral axillary adenopathy in the vaccination arm was the second most frequently reported local reaction, with 11.6% of recipients aged 18 to 64 reporting it after the first dose and 16.0% reported it after the second. The average duration of this lymphadenopathy was 1 to 2 days.
For the Pfizer-BioNTech COVID-19 vaccine, the CDC noted that reports of adenopathy were unbalanced between the vaccine and placebo groups and concluded that the adenopathy was plausibly related to the vaccine.
The mean duration of adenopathy was approximately 10 days.
The CDC notes that the adenopathy was reported within two to four days after vaccination for both vaccine groups.
However, the details of the cases reported by Dodelszon and colleagues draw a slightly different picture. For example, in case 1, the patient was diagnosed with unilateral axillary lymphadenopathy 9 days after receiving the first dose of the Pfizer-BioNTech vaccine. In case 3, the time elapsed between the Modern vaccine and the detection of adenopathy was 13 days.
In both cases, the time was much longer than the average duration of 1 to 2 days indicated by the CDC. The authors suggest that when taking the patient’s vaccination history, radiologists understand that the side effect may occur up to several weeks after vaccination against COVID-19.
In cases 2 and 4, axillary adenopathy was observed incidentally during mammography, so it is unclear when this reaction occurred after receiving the COVID-19 vaccine.
The authors and Leung have not disclosed any relevant financial relationship.
Clinical image. Published online January 18, 2021. Full text
For more information on Medscape Oncology, join us Twitter and Facebook.