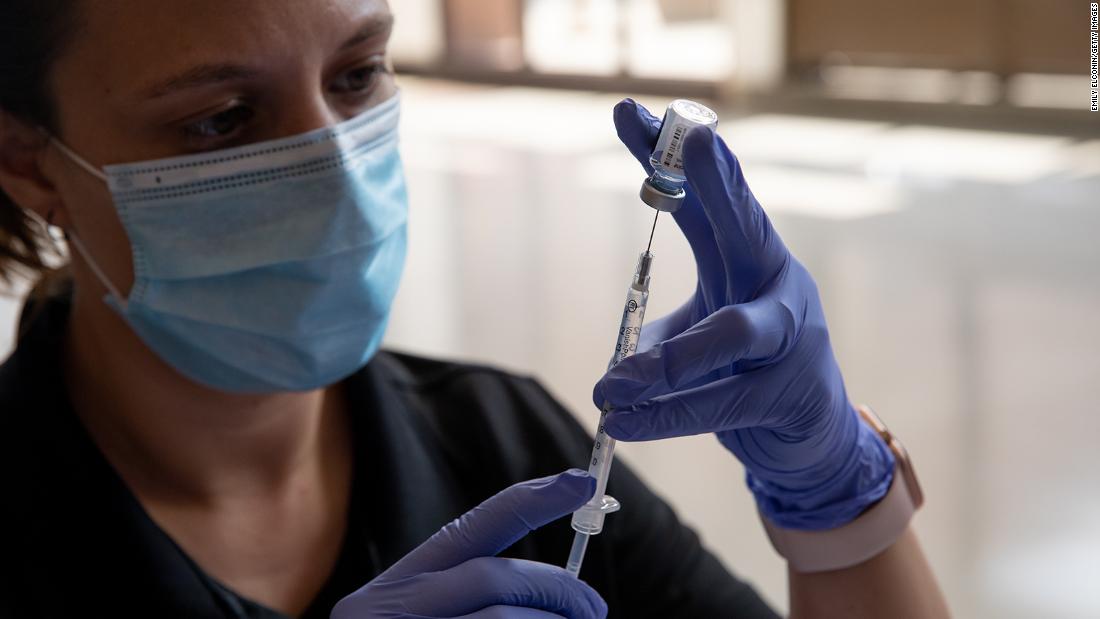
Federal health officials reported that Moderna’s presentation “was inadequate and needed enhanced data” from the company, a source said.
The Pfizer process continues on track, with the meeting of the FDA Advisory Committee on Vaccines and Related Biological Products set for Sept. 17, the source said.
Modern announced Wednesday that it has begun sending booster data to the FDA.
“These things happen and are part of the process,” the source said. “We have and will continue to follow science.”
“We have always said that we would follow science and all this is part of a process that is underway. We are waiting for a full review and approval by the FDA and a recommendation from the (Advisory Committee on Immunization Practices). approval and a recommendation is made, we will be prepared to implement the plan that the best doctors in our nation have developed so that we are ahead of this virus, ”Meagher said in a statement.
The FDA is already evaluating data submitted by Pfizer / BioNTech to approve a booster dose. Modern said Wednesday it began sending data to the FDA to support a booster dose of its vaccine to people six months after its second dose.
The FDA does not yet have enough data on Covid-19 booster shots, FDA Acting Commissioner Dr. Janet Woodcock said Thursday, despite the Sept. 20 White House’s scheduled date.
“Why would you announce that? Well, we need to have a plan and the plan would involve vaccinating a very large number of people in the United States with booster doses,” Woodcock told WebMD Dr. John Whyte during a virtual interview. posted Thursday online.
“We have to make a plan a little bit before we have all the data and I think, John, that’s what confuses people,” Woodcock said.
Woodcock added that while the FDA does not yet have all the data it needs on booster doses, studies will arrive soon.
“It’s true we don’t have all the data,” Woodcock said. “We don’t have all the data on the boost, all the safety data, etc. These studies have been completed and should be available to the FDA soon.”
It is likely that three doses of Modern or Pfizer coronavirus vaccine will end up being the recommended complete regimen to protect people from Covid-19 infection, Dr. Anthony Fauci said Thursday, stressing that it is up to the FDA to make the decision. final.
Fauci pointed to two Israel-based studies that showed a decrease in infections among people who received a third shot.
“But I have to say from my own experience as an immunologist, I wouldn’t be surprised at all that the proper regimen suitable for vaccination is three doses,” said Fauci, who is director of the National Institutes of Health. allergies and Infectious Diseases. at the Covid-19 White House response team briefing.
Waiting a few months between doses allows the immune system to develop a complete, mature response, which is then helped with a boost, he said.
“It’s entirely understandable why the results I just reported from the Israeli impetus are so dramatic,” Fauci said. “And we all hope and think we have good reason to believe that this will not only be a strong response, but will actually be lasting, and if it is lasting, you are likely to have three doses the regimen is the routine regimen.”
It relied heavily on the data Moderna and Pfizer present to support their FDA applications, he said.
If the FDA and the CDC Advisory Committee on Immunization Practices endorse a booster dose, the federal government will promote it, Zients said.
“Once the FDA and ACIP make their recommendations on boosters, the same intensity of operations, coordination and partnership will apply to the reinforcement campaign,” Zients said.
Johnson & Johnson is also studying the possibility of adding a second dose as a booster to the single vaccine.
This story has been updated with additional details on Friday.