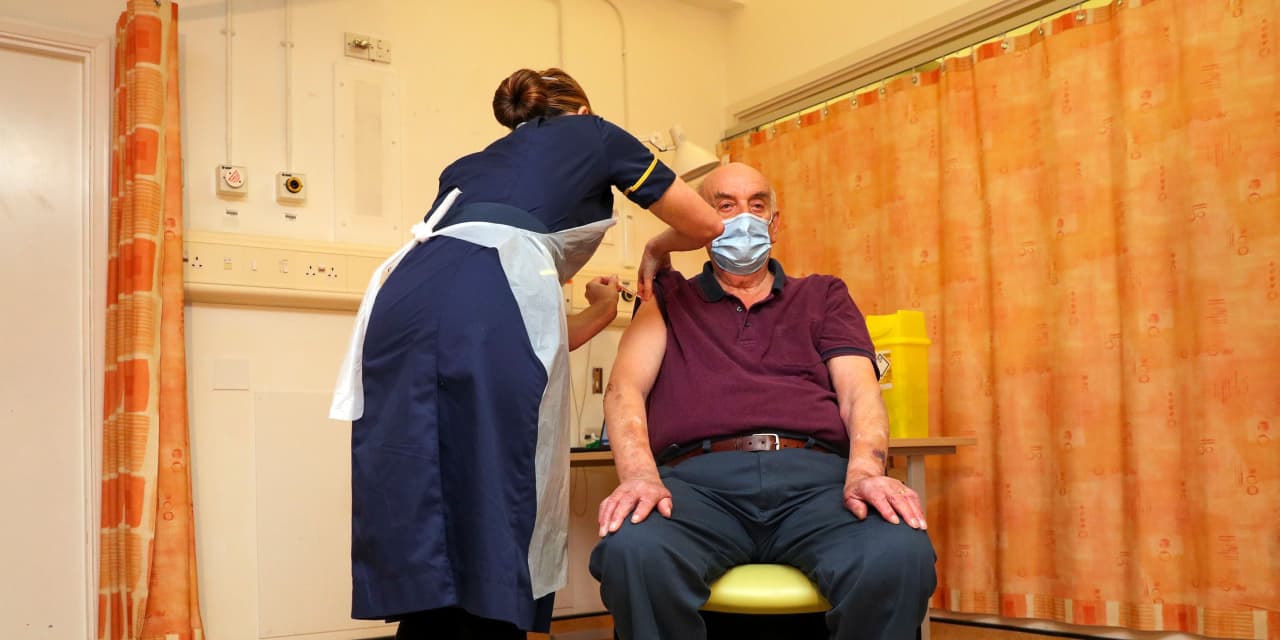
An 82-year-old dialysis patient became the first person in the world to receive the COVID-19 vaccine developed by AstraZeneca AZN on Monday.
and Oxford University since its use was approved in the UK, which is fighting a rapidly rising number of coronavirus cases.
Brian Pinker, a retired maintenance manager, was shot at 7.30am GMT by Nurse Sam Foster at Oxford Churchill Hospital. “I am very pleased to receive the vaccine against COVID today and I am very proud that it was invented in Oxford,” Pinker said in a statement issued by the National Health Service.
More than half a million doses of the vaccine from the pharmaceutical company AstraZeneca AZN,
and the Oxford University shot will be available from Monday, with tens of millions more to be delivered in the coming weeks and months once the regulator has verified the quality of the lots, the government said.
The UK government has secured access to 100 million doses of the AstraZeneca-Oxford vaccine, which was authorized for emergency use by the Medicines and Health Products Regulatory Agency (MHRA), the December 30th.
The shots will be delivered to 730 vaccination sites already established across the UK, and others will open this week to bring the total to more than 1,000, the government said in a statement.
“This is a crucial moment in our fight against this terrible virus and I hope it gives everyone a new hope that the end of this pandemic is in sight,” Health Secretary Matt Hancock said.
Hancock’s comments come almost a month after the UK began launching the vaccine developed by the American pharmaceutical company Pfizer PFE,
and its German partner BioNTech BNTX,
with over a million people receiving the first dose.
Last week, the MHRA, the Joint Vaccination and Vaccination Committee and four UK medical chiefs agreed to delay the gap between the first and second doses of vaccines, to try to protect as many people as possible in the shortest possible time.
The AstraZeneca – Oxford vaccine is easier to transport and store than the Pfizer – BioNTech vaccine, which must be kept below 70 degrees until shortly before use, making it easier to deliver to nursing homes. .
The launch of the AstraZeneca-Oxford vaccine comes amid a recurrent outbreak of coronavirus cases in the UK, with more than 50,000 new coronavirus cases recorded for the sixth day in a row. 54,990 new infections and 454 deaths were reported on Sunday, according to government data.
Prime Minister Boris Johnson told the national radio station BBC that tougher measures could be needed in some parts of the country in the coming weeks to control the rapid spread of the coronavirus caused by COVID-19. “If you look at the numbers, there’s no doubt that we’ll have to take tougher action and announce them in time,” Johnson said. On Monday he will set out plans for England in a televised speech at 8pm GMT.
Read: The slow deployment of the COVID-19 vaccine in the US could portend more problems
Earlier Monday, Scottish Prime Minister Nicola Sturgeon announced new blockade rules, with a legal requirement for everyone in mainland Scotland to stay home, except for essential purposes, from midnight tonight until the end of gener.
“As a result of this new variant, [the virus] he has just learned to run much faster and has certainly picked up pace in recent weeks, ”Sturgeon told the Scottish Parliament.
Meanwhile, some European Union leaders have been criticized for the slow pace of their vaccination programs, which began on December 27 with the launch of Pfizer – BioNTech.
BioNTech chief executive Uğur Şahin told German newspaper Der Spiegel that the process in Europe “was certainly not as quick and easy” as in other countries, in part because the EU is not directly authorized and member states they have their opinion.
Read: Why the failed vaccination campaign against COVID in France is seen as a failure of Macron and the failure of the ruling elite
The French government has pledged to speed up the pace of vaccinations after inoculating just over 350 people with the Pfizer – BioNtech shot in the first six days, compared to Germany’s 238,000. Starting Monday, medical staff aged 50 and over in France will receive the shots. Vaccines in the Netherlands will not be administered until January 8, when the computer system needed to plan and record shots will be ready.
Several European countries are expected to extend the blockades amid rising coronavirus cases. On Saturday, France moved to a night curfew in 15 departments from 8pm to 6pm. German Chancellor Angela Merkel will meet with German heads of state on Tuesday to decide whether to extend the current closure beyond 10 January.